You are in: eMedicine Specialties > Neurology > Movement And Neurodegenerative Diseases
|
Vitamin B-12 Associated Neurological Diseases Last Updated: July 18, 2006 |
|
| Synonyms and related keywords: vitamin B-12 deficiency, cobalamin deficiency, subacute combined spinal degeneration, pernicious anemia, PA, vitamin B-12 associated neurologic diseases, vitamin B-12 associated neurological diseases
|
| |
AUTHOR INFORMATION
| Section 1 of 11   |
|
| Author: Niranjan N Singh, MD, DM, DNB, Staff Physician, Department of Neurology, St Louis University Hospital Coauthor(s): Florian P Thomas, MD, MA, PhD, DrMed, Associate Chief of Staff, St Louis VA Medical Center; Associate Director, Neurology Residency Program; Professor, Departments of Neurology, Molecular Virology, and Molecular Microbiology and Immunology, Saint Louis University School of Medicine; Alan L Diamond, DO, Movement Disorder Fellow, Department of Neurology, Baylor College of Medicine; René Diamond, DO, Resident, Internal Medicine, Saint Louis University School of Medicine |
| Niranjan N Singh, MD, DM, DNB, is a member of the following medical societies:
American Academy of Neurology, and
American Medical Association |
| Editor(s): Christopher C Luzzio, MD, Clinical Assistant Professor, Department of Neurology, University of Wisconsin at Madison; Francisco Talavera, PharmD, PhD, Senior Pharmacy Editor, eMedicine;
Nestor Galvez-Jimenez, MD, Program Director of Movement Disorders, Director of Neurology Residency Training Program, Department of Neurology, Division of Medicine, Cleveland Clinic Florida;
Selim R Benbadis, MD, Professor of Neurology, Director of Comprehensive Epilepsy Program, Departments of Neurology and Neurosurgery, University of South Florida College of Medicine, Tampa General Hospital;
and Nicholas Lorenzo, MD, Chief Editor, eMedicine Neurology; Consulting Staff, Neurology Specialists and Consultants |
Disclosure
| |
INTRODUCTION
| Section 2 of 11    |
|
Background: The association of anemia and gastrointestinal and neurologic abnormalities referable to the brain, spinal cord, and peripheral nerves has been recognized in several clinical and postmortem case reports and series by Combe, Addison, and Fenwick since the early 19th century. In 1877, Gardner and Osler coined the term pernicious anemia (PA) to describe a patient with progressive arm numbness and difficulty with buttoning and using tools. Liechtenstein in 1884 reported the association of PA and spinal cord disease but attributed both to tabes dorsalis. Lichtheim in 1887 and Minnich in 1892 recognized the histologic differences in the spinal cord between PA and tabes dorsalis.
In 1900, Russell et al coined the term subacute combined degeneration of the spinal cord. In 1926, Minot and Murphy fed PA patients a half-pound of calf liver daily, for which they received the Nobel Prize. In 1929, Castle distinguished the role of gastric (intrinsic) and dietary (extrinsic) factors in PA. In 1948, cyanocobalamin was isolated from the liver. The existence of vitamin B-12 deficiency neuropathy was recognized in 1958. In 1955, Lassen et al noted megaloblastic anemia secondary to prolonged nitrous oxide (N2O) exposure; the neurologic features were described in 1978 by Sahenk et al and Layzer et al. Pathophysiology:
Vitamin B-12 structure
Vitamin B-12 (cobalamin) is a complex molecule in which a cobalt atom is contained in a corrin ring. Vitamin B-12 is available in animal protein.
Body stores
Total body stores are 2-5 mg, of which half is stored in the liver. The recommended daily intake is 2 mcg/d in adults; pregnant and lactating women require 2.6 mcg/d. Children require 0.7 mcg/d and, in adolescence, 2 mcg/d. Because vitamin B-12 is highly conserved through the enterohepatic circulation, cobalamin deficiency from malabsorption develops after 2-5 years and deficiency from dietary inadequacy in vegetarians develops after 10-20 years. Its causes are mainly nutritional and malabsorptive, PA being most common.
Physiology of absorption
After ingestion, the low stomach pH cleaves cobalamin from other dietary protein. The free cobalamin binds to gastric R binder, a glycoprotein in saliva, and the complex travels to the duodenum and jejunum, where pancreatic peptidases digest the complex and release cobalamin. Free cobalamin can then bind with gastric intrinsic factor (IF), a 50-kd glycoprotein produced by the gastric parietal cells, the secretion of which parallels that of hydrochloric acid. Hence, in states of achlorhydria, IF secretion is reduced, leading to cobalamin deficiency. Importantly, only 99% of ingested cobalamin requires IF for absorption. Up to 1% of free cobalamin is absorbed passively in the terminal ileum. This why oral replacement with large vitamin B-12 doses is appropriate for PA.
Once bound with IF, vitamin B-12 is resistant to further digestion. The complex travels to the distal ileum and binds to a specific mucosal brush border receptor, cublin, which facilitates the internalization of cobalamin-IF complex in an energy-dependent process. Once internalized, IF is removed and cobalamin is transferred to other transport proteins, transcobalamin I, II, and III (TCI, TCII, TCIII). Eighty percent of cobalamin is bound to TCI/III, whose role in cobalamin metabolism is unknown. The other 20% binds with TCII, the physiologic transport protein produced by endothelial cells. Its half-life is 6-9 min, thus delivery to target tissues is rapid.
The cobalamin-TCII complex is secreted into the portal blood where it is taken up mainly in the liver and bone marrow as well as other tissues. Once in the cytoplasm, cobalamin is liberated from the complex by lysosomal degradation. An enzyme-mediated reduction of the cobalt occurs by cytoplasmic methylation to form methylcobalamin or by mitochondrial adenosylation to form adenosylcobalamin, the 2 metabolically active forms of cobalamin.
Vitamin B-12 role in bone marrow function
In the cytoplasm, methylcobalamin (see Image 1) serves as cofactor for methionine synthesis by allowing transfer of a methyl group from 5-methyl-tetrahydrofolate (5-methyl-THF) to homocysteine (HC), forming methionine and demethylated tetrahydrofolate (THF). This results in reduction in serum homocysteine, which appears to be toxic to endothelial cells. Methionine is further metabolized to S-adenosylmethionine (SAM).
THF is used for DNA synthesis. After conversion to its polyglutamate form, THF participates in purine synthesis and the conversion of deoxyuridylate (dUTP) to deoxythymidine monophosphate (dTMP), which is then phosphorylated to deoxythymidine triphosphate (dTTP). dTTP is required for DNA synthesis; therefore, in vitamin B-12 deficiency, formation of dTTP and accumulation of 5-methyl-THF is inadequate, trapping folate in its unusable form and leading to retarded DNA synthesis. RNA contains dUTP (deoxyuracil triphosphate) instead of dTTP, allowing for protein synthesis to proceed uninterrupted and resulting in macrocytosis and cytonuclear dissociation.
Because folate deficiency causes macrocytosis and cytonuclear dissociation via the same mechanisms, both deficiencies lead to megaloblastic anemia and disordered maturation in granulocytic lineages; therefore, folate supplementation can reverse the hematologic abnormalities of vitamin B-12 deficiency but has no impact on the neurologic abnormalities of vitamin B-12 deficiency, indicating both result from different mechanisms.
Vitamin B-12 role in the peripheral and central nervous systems
The neurologic manifestation of cobalamin deficiency is less well understood. CNS demyelination may play a role, but how cobalamin deficiency leads to demyelination remains unclear. Reduced SAM or elevated methylmalonic acid (MMA) may be involved.
SAM is required as the methyl donor in polyamine synthesis and transmethylation reactions. Methylation reactions are needed for myelin maintenance and synthesis. SAM deficiency results in abnormal methylated phospholipids such as phosphatidylcholine, and it is linked to central myelin defects and abnormal neuronal conduction, which may account for the encephalopathy and myelopathy. In addition, SAM influences serotonin, norepinephrine, and dopamine synthesis. This suggests that, in addition to structural consequences of vitamin B-12 deficiency, functional effects on neurotransmitter synthesis that may be relevant to mental status changes may occur. Parenthetically, SAM is being studied as a potential antidepressant.
Another possible cause of neurologic manifestations involves the other metabolically active form of cobalamin, adenosylcobalamin (see Image 2), a mitochondrial cofactor in the conversion of L-methylmalonyl CoA to succinyl CoA. Vitamin B-12 deficiency leads to an increase in L-methylmalonyl-CoA, which is converted to D-methylmalonyl CoA and hydrolyzed to MMA. Elevated MMA results in abnormal odd chain and branched chain fatty acids with subsequent abnormal myelination, possibly leading to defective nerve transmission.
More recent studies propose a very different paradigm: B-12 and its deficiency impact a network of cytokines and growth factors, ie, brain, spinal cord, and CSF TNF-alpha; nerve growth factor (NGF), IL-6 and epidermal growth factor (EGF), some of which are neurotrophic, others neurotoxic. Vitamin B-12 regulates IL-6 levels in rodent CSF. In rodent models of B-12 deficiency parenteral EGF or anti-NGF antibody injection prevents, like B-12 itself, the SCD-like lesions.
In the same models, the mRNAs of several cell-type specific proteins (glial fibrillary acidic protein, myelin basic protein) are decreased in a region specific manner in the CNS, but, in the PNS myelin, protein zero and peripheral myelin protein 22 mRNA remain unaltered.
In human and rodent serum and CSF, concomitantly with a vitamin B-12 decrease, EGF levels are decreased, while at the same time, TNF-alpha increases in step with homocysteine levels. These observations provide evidence that the clinical and histological changes of vitamin B-12 deficiency may result from up-regulation of neurotoxic cytokines and down-regulation of neurotrophic factors.
NO pathomechanisms in vitamin B-12 deficiency
NO can oxidize the cobalt core of vitamin B-12 from a 1+ to 3+ valance state, rendering methylcobalamin inactive, inhibiting HC conversion to methionine and depleting the supply of SAM. Patients with sufficient vitamin B-12 body stores can maintain cellular functions after NO exposure, but in patients with borderline or low vitamin B-12 stores, this oxidation may be sufficient to precipitate clinical manifestations. Frequency:
- In the US: The prevalence of vitamin B-12 deficiency is difficult to ascertain because of diverse etiologies and different assays, ie, radioassay or chemiluminescence. Affected individuals may number 300,000 to 3 million in the United States.
Using the radioassay and a value less than 200 pg/mL, the prevalence of vitamin B-12 deficiency is 3-16%. In a geriatric population using a radioassay cutoff of 300 pg/mL and elevated HC and MMA levels, a prevalence of 21% was reported.
Eleven percent of HIV-seropositive individuals are vitamin B-12 deficient; another 12% have levels of 200-240 pg/mL. In a subgroup with chronic diarrhea, the rate reaches 39%. However, the importance for vitamin B-12 deficiency in the development of neurologic disease in these patients remains unclear.
Mortality/Morbidity: - Vitamin B-12 deficiency is associated with an elevated HC.
- The prevalence of hyperhomocysteinemia in the general population is 5-10%; in people older than 65 years it may reach 30-40%. Elevated HC is a risk factor for coronary artery, cerebrovascular, and peripheral vascular diseases and venous thrombosis. About 10% of the vascular disease risk in the general population is linked to HC.
- Case-control studies have reported a correlation between multi-infarct dementia or dementia of the Alzheimer type and elevated HC; vitamin B-12 supplementation had no clinical benefit.
- Neural tube defects are associated with low folate and vitamin B-12.
- PA patients have a 3 times and 13 times increased risk of gastric carcinoma and gastric carcinoid tumors, respectively.
- Patients with diabetes mellitus type 1 and autoimmune thyroid diseases are at higher risk of developing PA.
- Multifactorial abnormalities of vitamin B-12 metabolism and absorption occur in HIV infection.
Race:
- PA prevalence may be higher in white people and lower in Hispanic and black people.
- No known relationship exists between neurologic symptoms and race.
- Studies in Africa and the United States have shown higher vitamin B-12 and transcobalamin II levels in black than in white individuals. Additionally, blacks have lower HC levels and metabolize it more efficiently than whites.
Sex:
- In Europe and Africa, the prevalence of PA is higher in elderly women than men (1.5:1), while in the United States no differences exist.
- Men have higher HC levels at all ages.
- Pregnancy and estrogen replacement in postmenopausal women lower HC levels.
Age:
- PA occurs in people of all ages, but it is more common in people older than 40-70 years and, in particular, in people older than 65 years.
- In white people, the mean age of onset is 60; in black people, the mean age is 50 years.
- Congenital PA manifests in children aged 9 months to 10 years; the mean age is 2 years.
History:
Clinical course
The neurologic features are attributable to pathology in the peripheral and optic nerves, posterior and lateral columns of the spinal cord (subacute combined degeneration), and in the brain. Interestingly, hematologic and neurologic manifestations are occasionally dissociated. An inverse correlation in the severity of both manifestations has been suggested. In patients with neuropsychiatric abnormalities, 28% lack anemia or macrocytosis.
Although the clinical features of vitamin B-12 deficiency may consist of a classic triad of weakness, sore tongue, and paresthesias, these are not usually the chief symptoms.
Onset is subacute or gradual, although more acute courses have been described, in particular after NO exposure. In 1986, Schilling described 2 patients with unrecognized vitamin B-12 deficiency who developed paresthesias and poor manual dexterity 1-3 months after brief NO exposure. In 1995, Kinsella and Green described a 70-year-old man with paresthesias and hand clumsiness after 2 exposures to NO over 3 months.
Onset is often with a sensation of cold, numbness, or tightness in the tips of the toes and then in the fingertips, rarely with lancinating pains. Simultaneous involvement of arms and legs is uncommon, and onset in the arms is even rarer.
Paresthesias are ascending and occasionally involve the trunk, leading to a sensation of constriction in the abdomen and chest.
Untreated patients may develop limb weakness and ataxia.
In 1991, Healton et al performed detailed neurologic evaluations of 143 patients with vitamin B-12 deficiency. Seventy-four percent presented with neurologic symptoms. - Isolated numbness or paresthesias were present in 33%.
- Gait abnormalities occurred in 12%.
- Psychiatric or cognitive symptoms were noted in 3%.
- Visual symptoms were reported in 0.5%. Symptoms include subacute progressive decrease in visual acuity, usually caused by bilateral optic neuropathy and rarely pseudotumor cerebri or optic neuritis.
- Rare autonomic features include orthostasis, sexual dysfunction, and bowel and bladder incontinence.
- Other symptoms include lightheadedness and impaired taste and smell.
- Asymptomatic neurologic manifestations can be detected using somatosensory evoked potentials (SSEP); see below.
- Nonneurologic symptoms, some of which may also reflect autonomic nervous system involvement, were present in 26%.
- Constitutional symptoms, including anorexia and weight loss occurred in 50%. Low-grade fever that resolves with treatment occurred in 33% of cases. Other symptoms include fatigue and malaise.
- Cardiovascular symptoms include syncope, dyspnea, orthopnea, palpitations, and angina.
- Gastrointestinal symptoms include heartburn, flatulence, constipation, diarrhea, sore tongue, and early satiety.
Physical: Most patients exhibit signs of peripheral nervous system (PNS) or spinal cord involvement, but the extent of PNS involvement remains unclear, in part because both neuropathy and myelopathy can cause impaired vibration sense, ataxia, and paresthesias. Either can be affected first in the early stages. Objective sensory abnormalities usually result from posterior column involvement and less often from PNS disease.
In 1919, Woltman found features of PNS disease in 4.9% of patients with PA, including distal hyporeflexia or areflexia; 80% of these also had evidence of cord involvement. - In 1991, Healton summarized his experience with a large group of patients as follows:
- Isolated neuropathy was reported in 25% of patients.
- Myelopathy occurred in 12 % of cases.
- A combination of neuropathy and myelopathy was noted in 41%.
- Neuropsychiatric manifestations, such as recent memory loss with reduced attention span and otherwise normal cognition, depression, hypomania, paranoid psychosis with auditory or visual hallucinations (megaloblastic madness), violent behavior, personality changes, blunted affect, and emotional liability, were reported in 8% of patients.
- Ocular findings included a cecocentral scotoma and occurred in 0.5% of cases. Others have described optic atrophy, nystagmus, small reactive pupils, and chiasmatic lesion causing bitemporal hemianopia.
- Normal findings were noted on neurologic examination in 14% of patients despite paresthetic symptoms.
- Early in the course, poor joint position and vibration sense predominate. Typically, the legs are affected before the arms. Rarely are all limbs affected simultaneously. A Romberg sign is commonly found. The gait may be wide based.
- On presentation, 50% of patients have absent ankle reflexes with relative hyperreflexia at the knees. Plantars are initially flexor and later extensor. A Hoffman sign may be found.
- As the disease progresses, ascending loss of pinprick, light touch, and temperature sensation occurs. Later, depending on the predominance of posterior column versus cortical spinal tract involvement, ataxia or spastic paraplegia predominates. Then, PNS involvement causes distal limb atrophy.
- Cognitive testing may reveal mild impairment or frank dementia.
- Nonneurologic manifestations include the following:
- General - Lemon-yellow waxy pallor, premature whitening of hair, flabby bulky frame, mild icterus, and blotchy skin pigmentation in dark-skinned patients
- Cardiovascular - Tachycardia, congestive heart failure
- Gastrointestinal - Beefy, red, smooth, and sore tongue with loss of papillae that is more pronounced along edges
- Abnormal vitamin B-12 metabolism occurs in infants born to vitamin B-12–deficient mothers or those with hereditary diseases, including the Imerslünd-Grasbeck syndrome (cublin mutation resulting in decreased cobalamin transport from the intestinal lumen), transcobalamin II deficiency, and intracellular cobalamin abnormalities (classified as Cbl A though G with neurologic features in Cbl C and Cbl D, see below). Symptoms become prominent after exhaustion of vitamin B-12 stores acquired in utero. Infants present with developmental delay, failure to thrive, lethargy, poor feeding, mental retardation, seizures, listlessness, irritability, ataxia, hyporeflexia, hypotonia, pathologic reflexes, coma, tremor, and myoclonus. The latter may worsen transiently upon initiation of treatment.
Causes: Inadequate vitamin B-12 absorption is the major pathomechanism and may result from several factors. - Intrinsic factor deficiency
- PA accounts for 75% of cases of vitamin B-12 deficiency. It is an autoimmune attack on gastric IF. Antibodies are present in 70% of patients. They may block the formation of the cobalamin-IF complex or block its binding with cublin. Other antibodies are directed at parietal cell hydrogen-potassium adenosine triphosphatase (ATPase).
- Juvenile PA results from inability to secrete IF. Secretion of hydrogen ions and the gastric mucosa are normal. Transmittance is autosomal recessive inheritance of abnormal GIF on chromosome arm 11q13.
- Destruction of gastric mucosa can occur from gastrectomy or Helicobacter pylori infection. A Turkish study found endoscopic evidence of H pylori infection in more than 50% of vitamin B-12–deficient patients. Antibiotics alone eradicated H pylori in 31 patients, with resolution of vitamin B-12 deficiency.
- Deficient vitamin B-12 intake: Intake may be inadequate because of strict vegetarianism (rare), breastfeeding of infants by vegan mothers, alcoholism, or following dietary fads.
- Disorders of terminal ileum: Tropical sprue, celiac disease, enteritis, exudative enteropathy, intestinal resection, Whipple disease, ileal tuberculosis, and cublin gene mutation on chromosome arm 10p12.1 in the region designated MGA 1, which affects binding of the cobalamin-IF complex to intestinal mucosa (Imerslünd-Grasbeck syndrome), are disorders that affect the terminal ileum.
- Competition for cobalamin: Competition for cobalamin may occur in blind loop syndrome or with fish tapeworm (Diphyllobothrium latum).
- Abnormalities related to protein digestion related to achlorhydria: Abnormalities include atrophic gastritis, pancreatic deficiency, proton pump inhibitor use, and Zollinger-Ellison syndrome, in which the acidic pH of the distal small intestine does not allow the cobalamin-IF complex to bind with cublin.
- Medications: Medications include colchicine, neomycin, and p-aminosalicylic acid.
- Transport protein abnormality: Abnormalities include transcobalamin II deficiency (autosomal recessive inheritance of an abnormal TCN2 gene on chromosome arm 22q11.2-qter resulting in failure to absorb and transport cobalamin) and deficiency of R-binder cobalamin enzyme.
- Disorders of intracellular cobalamin metabolism: These disorders result in methylmalonic aciduria and homocystinuria in infants.
- Isolated methylmalonic aciduria
- Cbl A is due to deficiency of mitochondrial cobalamin reductase resulting in deficiency of adenosylcobalamin.
- Cbl B is due to deficiency of adenosylcobalamin transferase resulting in deficiency of adenosylcobalamin.
- Methylmalonic aciduria and homocystinuria
- Cbl C is a combined deficiency of methylmalonyl CoA mutase and homocysteine:methyltetrahydrofolate methyltransferase. Patients have prominent neurologic features and megaloblastic anemia.
- Cbl D is a deficiency of cobalamin reductase. Patients have prominent neurologic features.
- Cbl F is a defect in lysosomal release of cobalamin.
- Isolated homocystinuria
- Cbl E is due to a defect in methionine synthase reductase located on chromosome arm 5p15.3-p15.2.
- Cbl G is due to a defect in methyltetrahydrofolate homocysteine methyltransferase located on chromosome arm 1q43.
- Increased vitamin B-12 requirement: Requirement is increased in hyperthyroidism and alpha thalassemia.
- In AIDS, vitamin B-12 deficiency is not infrequent. Although the exact etiology remains obscure, it is likely a multimodal process involving poor nutrition, chronic diarrhea, ileal dysfunction, and exudative enteropathy. Low vitamin B-12 levels may be more common in late than in early HIV disease.
- NO exposure can occur iatrogenically (ie, anesthesia) or through abuse ("whippets").
| |
DIFFERENTIALS
| Section 4 of 11    |
|
HIV-1 Associated Vacuolar Myelopathy
Lyme Disease
Multiple Sclerosis
Neurosyphilis
Toxic Neuropathy
Tropical Myeloneuropathies
Other Problems to be Considered:
Human T-cell leukemia virus type 1 (HTLV-1) myelopathy
Folate deficiency |
|
Continuing Education
|
| CME available for this topic. Click here to take this CME. |
|
|
Patient Education
|
|
Click here for patient education.
|
|
|
|
|
Lab Studies:
- Clinical evidence of vitamin B-12 deficiency
- Serum cobalamin levels are the initial test.
- Two assays exist: radioassay and the nonradioisotopic assay, chemiluminescence, which is becoming more popular because of improved automation, safety, and cost. Chemiluminescence has a higher reference range value, from 250-1100 pg/mL versus 170-900 pg/mL for radioassay. Using the radioassay and elevated HC and MMA as criterion standards, levels are less than 200 pg/mL in 90-95% of patients, 200-300 pg/mL in 5-10%, and greater than 300 pg/mL in 0.1-1%. Be aware of the assay used and how the reference range was determined. A serum cobalamin level that is within the reference range does not exclude cobalamin deficiency.
- Abnormally low vitamin B-12 levels: Test for PA by measuring antibodies against IF.
- Antiparietal cell antibodies are present in 90% of PA cases. Ten percent of patients older than 70 years have false-positive abnormal antibody levels.
- IF antibodies are present in 60% of patients. These are more specific but less sensitive.
- If either antibody is positive, the diagnosis of PA is confirmed and further testing is not required.
- If antibodies are negative, obtain a serum gastrin level to test for achlorhydria, which is associated with PA. If these are elevated, the diagnosis is likely PA. If these results are normal, perform a Schilling test (see below).
- Borderline vitamin B-12 level and clinical features of vitamin B-12 deficiency: Measure MMA and HC.
- Both folate and vitamin B-12 deficiency can lead to metabolite elevation.
- In vitamin B-12 deficiency, MMA and HC are elevated, although HC elevation occurs by itself. MMA is more sensitive than HC.
- In folate deficiency, MMA is within the reference range and HC is elevated.
- MMA and HC are considered abnormal when greater than 3 standard deviations above the mean. Reference range values are not age dependent for MMA and are 70-350 nM/L. For patients younger than 60 years, reference range values are 5-15 mM/L for HC. In people older than 60 years, the cutoff for HC is 20 mM/L.
- If both metabolites are within the reference range, vitamin B-12 deficiency is effectively ruled out. Only 0.2% of 400 patients with low serum vitamin B-12 had normal metabolite levels, and 10% of 98 patients with folate deficiency had metabolite levels within the reference range. False-positive elevations in MMA and HC occur in inborn errors of metabolism, renal disease, and deficiencies of folate. If either metabolite is elevated, test for PA or use the Schilling test.
- Schilling test: The Schilling test is used to determine the etiology of vitamin B-12 deficiency in patients with normal IF antibodies.
- Stage 1: Administer radiolabeled cobalamin 0.5-2.0 mCi PO to fasted patients. One to 6 hours later, administer unlabeled cobalamin 1000 mcg IM to saturate transcobalamin and flush hepatic storage. Measure the percentage of radiolabeled cobalamin in a 24-hour urine specimen. Urinary excretion within the reference range is 10-35% over 24 hours. Reduced urinary excretion of cobalamin, ie, less than 7-9% based on individual laboratory reference range values, in persons with normal renal function supports decreased absorption of oral cobalamin. If excretion is low, proceed to stage 2.
- Stage 2: Stage 1 is repeated with coadministration of porcine IF 60 mg. If the absorption of cobalamin is normalized, the presumptive diagnosis is PA. If poor absorption persists after administration, proceed to stage 3.
- Stage 3: Tetracycline is administered for 5 days prior to reperformance of stage 1 to exclude blind loop as the etiology.
- Stage 4: Pancreatic enzymes are administered with repetition of stage 1 to test for pancreatic disease.
- Caveats: If vitamin B-12 is administered 48 hours before the Schilling test, dilution of the radiolabeled cobalamin and spuriously low apparent urinary excretion and false-positive results occur. False-negative values occur in food-bound malabsorption due to achlorhydria. True negative results are from dietary deficiencies (vegan) and cobalamin binding–protein abnormalities.
- Routine hematologic and chemistry tests
- Hematologic abnormalities may be absent at the time of neurologic presentation.
- Vitamin B-12 deficiency produces the classic picture of macrocytic anemia, with a mean corpuscular value (MCV) greater than 100 fL. The MCV correlates with estimated vitamin B-12 level:
- MCV of 80-100 fL (normal) indicates less than 25% probability of vitamin B-12 deficiency.
- MCV of 115-129 fL indicates a 50% probability.
- MCV greater than 130 fL indicates a 100% probability.
- Peripheral blood smear shows macro-ovalocytosis, anisocytosis, and poikilocytosis, as well as basophilic stippling of the erythrocytes and Howell-Jolly bodies. Reticulocyte count can be within the reference range or low. Hypersegmentation (>5% of neutrophils with >5 lobes or 1% with >6 lobes) of polymorphonuclear cells may occur without anemia. Thrombocytopenia is observed in approximately 50% of patients, and platelets often have bizarre size and shape.
- Serum indirect bilirubin and lactate dehydrogenase (LDH) may be elevated because PA can have a hemolytic component.
- Achlorhydria is present in many patients with PA.
- Laboratory parameters after administration of vitamin B-12
- Anemic patients
- Reticulocytosis starts in 3-4 days and peaks at 1 week.
- Hemoglobin concentration rises in 10 days and returns to the reference range in 8 weeks.
- LDH falls within 2 days.
- Hypersegmented neutrophils disappear in 1-2 weeks.
- Patients with severe anemia and borderline-to-low iron stores
- Serum iron level falls within 24 hours because of increased erythropoiesis.
- Hypokalemia may develop because of increased potassium utilization in hematopoiesis.
Imaging Studies:
- Because of the increased incidence of gastric cancer in PA, gastric radiographic series are suggested at the first visit.
- In patients with myelopathy, MRI may reveal regional T2 and fluid-attenuated inversion recovery (FLAIR) hyperintensities mainly in the thoracic posterior columns with possible extension into the brain stem. In patients with chronic disease, atrophy of the spinal cord is observed.
- Brain MRI may show T2 and FLAIR hyperintensities in the cerebral white matter and around the fourth ventricle.
- Brain MRI of infants with vitamin B-12 deficiency may show delayed myelination.
Other Tests:
- Abnormal evoked potentials may be the first electrodiagnostic finding, even in asymptomatic patients with normal neurologic examination findings. The abnormalities are often referable to a central conduction defect; however, peripheral nerves are also affected.
- Somatosensory evoked potentials (SSEP) may reveal prolongation of L3-P27 latency, reflecting a defect in conduction in the large-fiber sensory pathway between the cauda equina and the contralateral sensory cortex.
- Visual evoked potential (VEP) findings are as follows:
- VEP findings may be abnormal even without visual symptoms or signs.
- Prolongation of the P100 waveform can be unilateral or bilateral.
- P100 may normalize after cobalamin replacement.
- Nerve conduction study (NCS)/EMG findings are as follows:
- In 1943, Dynes and Norcross found evidence of neuropathy in 23% of patients with PA.
- In a recent study, up to 65% of untreated patients had peripheral neuropathy.
- Axonal sensorimotor polyneuropathy is present in up to 80%. Demyelinating or mixed forms are less frequent.
- Typical features are decreased conduction velocities and motor or sensory amplitude and denervation on EMG.
- In 1991, Healton et al found decreased motor nerve conduction velocities (NCV), absent or reduced sensory potentials, and fibrillations in the distal muscles indicating mixed demyelinating-axonal disease in 7 of 9 cases of vitamin B-12 deficiency and neuropathy.
- In 1998, Steiner et al described 5 patients with demyelinating polyneuropathy.
- Sensory nerves are usually more affected than motor nerves and are more severely affected distally than proximally. Proximal focal conduction block has been reported, which reversed on treatment.
- Electroencephalography (EEG) findings may be normal or show nonspecific slowing. Follow-up EEG findings may be improved in response to treatment.
Procedures:
- Bone marrow aspiration may be performed for histologic examination.
Histologic Findings: The CNS is better characterized than the PNS in vitamin B-12 deficiency. The classic picture is subacute combined degeneration of the spinal cord involving the dorsal columns and corticospinal tracts. Lesions are concentrated in the cervical and upper thoracic cord and the cerebrum.
Spinal cord findings
- Macroscopic: It may be shrunken in anteroposterior diameter. The posterior and lateral columns may be gray-white in color.
- Microscopic: Multifocal vacuolated and demyelinated lesions exist in the white matter of the posterior and lateral columns. Early in the course, myelin sheaths are swollen, but axons appear normal; the largest fibers are predominantly affected. Demyelination starts in the center of the posterior columns of the upper thoracic cord. Then, lesions spread laterally and cranially to the lateral corticospinal tracts in the cervical segments and the medulla. Myelin breakdown, foamy macrophages, and occasional lymphocytes are characteristic, predominantly in a perivascular location. As demyelination and vacuolation increase, axons degenerate. Gliosis may be present in older lesions.
- Electronic microscopic findings have only been used in nonhuman primates, not in humans. In the rhesus monkey model, the posterior and lateral spinal cord shows myelin degeneration characterized by separation of myelin lamellae and formation of intramyelin vacuoles leading to complete destruction of the myelin sheath and later to degeneration and loss of axons and gliosis.
- Vitamin B-12 deficiency myelopathy is virtually indistinguishable from vacuolar myelopathy in AIDS, which is also characterized by vacuolation between the myelin sheaths accompanied by macrophage infiltration. However, the presence of multinucleated giant cells is characteristic of AIDS and not of vitamin B-12 deficiency.
Brain findings
- Macroscopic: The brain appears normal.
- Microscopic: Findings resemble the spinal cord pathology with scanty small perivascular foci of demyelination within the white matter featuring myelin swelling and axon degeneration. The optic nerve typically shows degeneration, predominantly in the papillomacular bundles.
Peripheral nervous system findings
- Unlike EMG/NCS findings, which indicate predominantly axonal or mixed axonal-demyelinating neuropathy, histologic examination suggests either primary demyelinating or axonal neuropathy with secondary demyelination. However, most studies were performed before the introduction of semithin, teased fiber, and electron microscopy analysis.
- A biopsy of the anterior tibial nerve recorded loss of myelin sheath and no loss of axons (Greenfield, 1934).
- A sural biopsy revealed loss of myelinated fibers and no demyelination in teased fibers, indicating secondary demyelination (McCombe, 1984).
- In 1959, Coers and Woolf found endplate and preterminal axon abnormalities in muscle biopsies of patients with cobalamin deficiency, suggesting axonal damage.
- The pathology is better described in animal models. In cobalamin-deficient rats, electron microscopy revealed intramyelin and endoneural edema, with no or minimal axonal damage (reversible on administration of cobalamin) in nerves, dorsal root ganglia, and the ventral and dorsal roots.
Nonneurologic findings
- Bone marrow hyperplasia
- Mild-to-moderate splenomegaly
- Increased iron deposits in the reticuloendothelial system
- Atrophy in all layers of the stomach, sparing the pyloroduodenal region
| |
TREATMENT
| Section 6 of 11    |
|
Medical Care: - Establish the diagnosis and etiology of vitamin B-12 deficiency and treat with adequate doses.
- The consequences of vitamin B-12 deficiency, encephalopathy, myelopathy, and peripheral and optic neuropathy require adequate medical care.
- Physical therapy and occupational therapy are needed to improve gait, balance, and arm function. Patients may require canes or a walker for ambulation and safety.
- In patients with encephalopathy, neuropsychological interventions may improve cognition, social functioning, and interpersonal relationships.
- Patients with PA are at increased risk for gastric carcinoma, colorectal adenocarcinoma, and carcinoid tumors and must be monitored.
Consultations: Consultations with a gastroenterologist, a hematologist, and a neurologist must be considered. Diet: When the cause of vitamin B-12 deficiency is low intake, recommend that patients eat food that contains vitamin B-12 such as meat, eggs, cheese, and yogurt. Supplementation is required when religious or cultural restrictions render dietary changes impossible. Activity: In most patients with vitamin B-12–associated neuropathy/myelopathy, no restriction on physical activity is necessary unless weakness or gait ataxia is severe. Also, severe encephalopathy may lead to 24-hour supervision. In severe anemia or congestive heart failure, the patient should limit strenuous exercise.
| |
MEDICATION
| Section 7 of 11    |
|
Standard treatment in patients with vitamin B-12 deficiency consists of parenteral or oral cobalamin. The hematologic abnormalities may respond to folate, but the neurologic manifestations only respond to cobalamin.
Numerous treatment regimens have been proposed, including cobalamin 1000 mcg IM/SC daily for 5 days followed by 1000 mcg/wk for 5 weeks, then 100-1000 mcg/mo for life.
Because 1% of cobalamin is absorbed by passive diffusion, administration of large oral doses is an alternative; 1000 mcg daily yields a daily absorption of 10 mcg, which exceeds the 2-mcg recommended daily allowance (RDA) requirement.
In addition to cobalamin replacement, oral IF supplementation is being evaluated. Supplementation with SAM or methionine-rich diets are being studied for NO-induced myeloneuropathies.
Diagnosis and treatment of tapeworm infection and celiac and Crohn diseases can improve intestinal vitamin B-12 malabsorption. With blind loop syndrome, tetracycline can normalize the intestinal flora and vitamin B-12 absorption.
Drug Category: Dietary supplements -- Cyanocobalamin is used to replenish the deficiency caused by any of the etiologies described. Drug Name
| Cyanocobalamin or vitamin B-12 (Berubigen, Cyanoject) -- Most stable and available form of vitamin B-12. Absorbed rapidly to the organism from IM or SC applications.
Oral cyanocobalamin can replace parenteral formulations. Is effective in PA because 1% of free cobalamin is absorbed via diffusion rather than requiring the presence of IF.| Adult Dose | Parenteral administration: 1000 mcg/d IM/SC for 5 d or 1000 mcg IM 2 times per wk for 2 wk, then 1000 mcg/wk IM/SC for 5 wk, then 100-1000 mcg IM/SC every mo
Oral administration: 1000-2000 mcg PO qd for life| Pediatric Dose | Parenteral administration:
1000 mcg IM qd for 2 wk; congenital disorders require lifelong replacement of 1000 mcg every mo
Oral administration:
<12 years: Not established
>12 years: Administer as in adults| Contraindications | Documented hypersensitivity; Leber disease, ie, hereditary optic nerve atrophy (patients may experience severe and swift optic atrophy when treated with vitamin B-12); severe megaloblastic anemia (hypokalemia and sudden death may occur) |
|---|
| Interactions | Colchicine, paraaminosalicylic acid, and excessive ingestion of alcohol may produce malabsorption of vitamin B-12; chloramphenicol may reduce cobalamin efficacy through interference with erythrocyte maturation |
|---|
| Pregnancy |
A - Safe in pregnancy
|
|---|
| Precautions | Pregnancy category C if dose exceeds RDA; very rarely patients report adverse effects (most commonly asthenia, headache, pruritus, diarrhea, and dizziness); serious reactions such as anaphylaxis and vascular thrombosis may occur; severe hypokalemia may result after correction of megaloblastic anemia because of increased cellular potassium requirements |
|---|
|
|---|
|
|---|
|
|---|
Drug Name
| Folic acid (Folvite) -- Folate supplementation can reverse the hematologic abnormalities, but the neurologic manifestations only respond to cobalamin. |
|---|
| Adult Dose | 1 mg PO/IM/SC qd |
|---|
| Pediatric Dose | <12 years: Not established
>12 years: 1 mg PO/IM/SC qd| Contraindications | Documented hypersensitivity |
|---|
| Interactions | Increase in seizure frequency and subtherapeutic levels of phenytoin reported when used concurrently |
|---|
| Pregnancy |
A - Safe in pregnancy
|
|---|
| Precautions | Pregnancy category C if dose exceeds RDA; benzyl alcohol may be contained in some products as a preservative (associated with a fatal gasping syndrome in premature infants); resistance to treatment may occur in patients with alcoholism and deficiencies of other vitamins |
|---|
|
|---|
| |
FOLLOW-UP
| Section 8 of 11    |
|
Further Inpatient Care:
- Once therapy is initiated, hospitalization is only required for patients with life-threatening anemia or with severe neurologic deficits requiring supervision or rehabilitation.
Further Outpatient Care:
- Patients with neurologic impairment may require additional care in skilled nursing units or rehabilitation facilities. Outpatient follow-up is required to ensure response to therapy.
In/Out Patient Meds:
- Cobalamin 100-1000 mcg/mo SC/IM, 1000 mcg/d PO is provided for lifelong maintenance.
- Compliance must be verified to avoid recurrence of symptoms.
Deterrence/Prevention:
- Relatives of patients with PA must be made aware of the increased familial incidence.
- Individuals with total gastrectomy, pancreatectomy, or atrophic gastritis should undergo periodic testing for vitamin B-12 deficiency.
- Testing vitamin B-12 (and folate) levels in elderly patients is good practice because of the high incidence of deficiencies. Asymptomatic deficiency should be worked up and treated.
- Strict vegetarians should supplement vitamin B-12 in their diets.
Complications:
- If left untreated, neurologic complications worsen.
- Severe anemia may lead to congestive heart failure.
- Incidence of atrophic gastritis, gastric carcinoma, and carcinoid tumors is increased in patients with PA.
- Patients with PA are at increased risk for other autoimmune disorders, such as myasthenia gravis, Lambert-Eaton myasthenic syndrome, type 1 diabetes mellitus, Hashimoto thyroiditis, hypogammaglobulinemia, vitiligo, and rheumatoid arthritis.
- Risk of neural tube defects is increased in untreated pregnant women.
Prognosis:
- Prior to institution of therapy, death would occur in approximately 2 years after onset.
- With treatment, prognosis remains guarded.
- Clinical improvement is most pronounced within the first 2 months of treatment and may continue for 6 months.
- Patients with motor involvement for longer than 6 months show minimal improvement with treatment.
- Dramatic improvement with therapy is rare in patients with subacute cognitive deficits and very low vitamin B-12 levels. Cognitive improvement is often subtle and slow. The prognosis is better in patients with cognitive deficits for less than 6 months.
- Acquired forms of vitamin B-12 deficiency have better prognosis than congenital forms.
| |
MISCELLANEOUS
| Section 9 of 11    |
|
Medical/Legal Pitfalls:
- The major pitfall in these disorders is delay in diagnosis, overlooking an existing low vitamin B-12 level, and failure to treat.
| |
PICTURES
| Section 10 of 11    |
|
| Caption: Picture 3. Vitamin B-12–associated neurological diseases. Pernicious anemia. Characteristic lemon-yellow pallor with raw beef tongue lacking filiform papillae. Photo from Forbes and Jackson with permission. |  |  View Full Size Image View Full Size Image |
 eMedicine Zoom View (Interactive!) eMedicine Zoom View (Interactive!) |
Picture Type: Photo |
| Caption: Picture 4. Vitamin B-12–associated neurological diseases. Fluid attenuated inversion recovery (Flair) MRI sequence in a patient with cobalamin deficiency and neuropsychiatric manifestations. Discrete areas of hyperintensities are present in the corona radiata. | 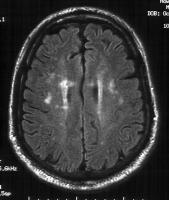 |  View Full Size Image View Full Size Image |
 eMedicine Zoom View (Interactive!) eMedicine Zoom View (Interactive!) |
Picture Type: MRI |
| |
BIBLIOGRAPHY
| Section 11 of 11    |
|
-
Adachi H, Hirai Y, Fujiura Y: Plasma homocysteine levels and atherosclerosis in Japan: epidemiological study by use of carotid ultrasonography. Stroke 2002 Sep; 33(9): 2177-81[Medline].
-
Addison T: Anemia: Disease of the suprarenal capsules. London Med Gazette 1849; 8: 517-518.
-
Al-Shubaili AF, Farah SA, Hussein JM, et al: Axonal and demyelinating neuropathy with reversible proximal conduction block, an unusual feature of vitamin B12 deficiency. Muscle Nerve 1998 Oct; 21(10): 1341-3[Medline].
-
Allen RH, Stabler SP, Savage DG: Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 1993 Nov; 7(14): 1344-53[Medline].
-
Andres E, Noel E, Kaltenbach G: [Vitamin B12 deficiency with normal Schilling test or non-dissociation of vitamin B12 and its carrier proteins in elderly patients. A study of 60 patients]. Rev Med Interne 2003 Apr; 24(4): 218-23[Medline].
-
Baik HW, Russell RM: Vitamin B12 deficiency in the elderly. Annu Rev Nutr 1999; 19: 357-77[Medline].
-
Balducci L: Epidemiology of anemia in the elderly: information on diagnostic evaluation. J Am Geriatr Soc 2003 Mar; 51(3 Suppl): S2-9[Medline].
-
Beach RS, Mantero-Atienza E, Shor-Posner G: Specific nutrient abnormalities in asymptomatic HIV-1 infection. AIDS 1992 Jul; 6(7): 701-8[Medline].
-
Berger JR, Quencer R: Reversible myelopathy with pernicious anemia: clinical/MR correlation. Neurology 1991 Jun; 41(6): 947-8[Medline].
-
Booth GL, Wang EE: Preventive health care, 2000 update: screening and management of hyperhomocysteinemia for the prevention of coronary artery disease events. The Canadian Task Force on Preventive Health Care. CMAJ 2000 Jul 11; 163(1): 21-9[Medline].
-
Boushey CJ, Beresford SA, Omenn GS, Motulsky AG: A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995 Oct 4; 274(13): 1049-57[Medline].
-
Carmel R, Johnson CS: Racial patterns in pernicious anemia. Early age at onset and increased frequency of intrinsic-factor antibody in black women. N Engl J Med 1978 Mar 23; 298(12): 647-50[Medline].
-
Carmel R, Johnson CS, Weiner JM: Pernicious anemia in Latin Americans is not a disease of the elderly. Arch Intern Med 1987 Nov; 147(11): 1995-6[Medline].
-
Carmel R: Reassessment of the relative prevalences of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: influence of patient age and race. Clin Exp Immunol 1992 Jul; 89(1): 74-7[Medline].
-
Carmel R: Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med 1996 May 27; 156(10): 1097-100[Medline].
-
Carmel R, Gott PS, Waters CH: The frequently low cobalamin levels in dementia usually signify treatable metabolic, neurologic and electrophysiologic abnormalities. Eur J Haematol 1995 Apr; 54(4): 245-53[Medline].
-
Castle WB: Extrinsic factor in pernicious anemia. American Journal of Medical Science 1929; 178: 148.
-
Chanarin I: The megaloblastic anemias. 2nd ed. Oxford, England: Blackwell Scientific; 1979.
-
Clarke R, Smith AD, Jobst KA, et al: Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 1998 Nov; 55(11): 1449-55[Medline].
-
Coers C, Woolf AL: The innervation of muscle. Oxford, England: Blackwell Scientific; 1959: 91.
-
Cole M: Neurological manifestation of vitamin B12 deficiency. In: Goetz C, Aminoff M, eds. Handbook of Clinical Neurology. Vol 26. Systemic Diseases. Part II. Amsterdam, Holland: Elsevier Science BV; 1998: 367-405.
-
Combe JJ: History of a case of anemia. Trans Med Chir Soc Edimb 1822; 1: 194-204.
-
Cunha UG, Rocha FL, Peixoto JM: Vitamin B12 deficiency and dementia. Int Psychogeriatr 1995 Spring; 7(1): 85-8[Medline].
-
Daly LE, Kirke PN, Molloy A, et al: Folate levels and neural tube defects. Implications for prevention. JAMA 1995 Dec 6; 274(21): 1698-702[Medline].
-
Dana CL: Subacute combined sclerosis of the spinal cord and its relation to anemia and to toxemia. J Nerv Ment Dis 1899; 26: 1.
-
Diaz-Arrastia R: Homocysteine and neurologic disease. Arch Neurol 2000 Oct; 57(10): 1422-7[Medline].
-
Dynes JB, Norcross JW: Peripheral neuritis as a complication of pernicious anemia. JAMA 1943; 122: 586-8.
-
Ehrenpreis ED, Carlson SJ, Boorstein HL: Malabsorption and deficiency of vitamin B12 in HIV-infected patients with chronic diarrhea. Dig Dis Sci 1994 Oct; 39(10): 2159-62[Medline].
-
Fenwick S: On atrophy of the stomach. Lancet 1870; ii: 78-80.
-
Fine EJ, Hallett M: Neurophysiological study of subacute combined degeneration. J Neurol Sci 1980 Mar; 45(2-3): 331-6[Medline].
-
Fine EJ, Soria E, Paroski MW: The neurophysiological profile of vitamin B12 deficiency. Muscle Nerve 1990 Feb; 13(2): 158-64[Medline].
-
Fowler B: Genetic defects of folate and cobalamin metabolism. Eur J Pediatr 1998 Apr; 157 Suppl 2: S60-6[Medline].
-
Gardner W, Osler W: A case of progressive pernicious anemia (Idiopathic of Addison). Can Med Surg J 1877; 5: 385-404.
-
Graham D, Lantos P: Vitamin Deficiencies. In: Greenfield's Neuropathology. 6th ed. London, England: Arnold Publishers; 1997: 621-624.
-
Grattan-Smith PJ, Wilcken B, Procopis PG: The neurological syndrome of infantile cobalamin deficiency: developmental regression and involuntary movements. Mov Disord 1997 Jan; 12(1): 39-46[Medline].
-
Green R, Kinsella LJ: Current concepts in the diagnosis of cobalamin deficiency. Neurology 1995 Aug; 45(8): 1435-40[Medline].
-
Greenfield JG: Subacute spinocerebellar degeneration occurring in elderly patients. Brain 1934; 57: 161-76.
-
Greenfield JG, Carmichael EA: Peripheral nerves in cases of subacute combined degeneration of the cord. Brain 1935; 58: 483-91.
-
Gueant JL, Saunier M, Gastin I: Decreased activity of intestinal and urinary intrinsic factor receptor in Grasbeck-Imerslund disease [corrected]. Gastroenterology 1995 Jun; 108(6): 1622-8[Medline].
-
Hamilton AS, Nixon CE: Sensory changes in the subacute combined degeneration of pernicious anemia. Arch Neurol Psychiatry 1921; 6: 1.
-
Healton EB, Savage DG, Brust JC: Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991 Jul; 70(4): 229-45[Medline].
-
Hemmer B, Glocker FX, Schumacher M, et al: Subacute combined degeneration: clinical, electrophysiological, and magnetic resonance imaging findings. J Neurol Neurosurg Psychiatry 1998 Dec; 65(6): 822-7[Medline].
-
Hennerici M: Dissociated foveal and parafoveal visual evoked responses in subacute combined degeneration. Arch Neurol 1985 Feb; 42(2): 130-2[Medline].
-
Hsing AW, Hansson LE, McLaughlin JK, et al: Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer 1993 Feb 1; 71(3): 745-50[Medline].
-
Hutto BR: Folate and cobalamin in psychiatric illness. Compr Psychiatry 1997 Nov-Dec; 38(6): 305-14[Medline].
-
Jacques PF, Rosenberg IH, Rogers G, et al: Serum total homocysteine concentrations in adolescent and adult Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr 1999 Mar; 69(3): 482-9[Medline].
-
Janson JJ, Galarza CR, Murua A: Prevalence of hyperhomocysteinemia in an elderly population. Am J Hypertens 2002 May; 15(5): 394-7[Medline].
-
Kagan BL, Sultzer DL, Rosenlicht N: Oral S-adenosylmethionine in depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry 1990 May; 147(5): 591-5[Medline].
-
Kang SS, Wong PW, Zhou JM, Cook HY: Total homocyst(e)ine in plasma and amniotic fluid of pregnant women. Metabolism 1986 Oct; 35(10): 889-91[Medline].
-
Kapadia CR: Vitamin B12 in health and disease: part I--inherited disorders of function, absorption, and transport. Gastroenterologist 1995 Dec; 3(4): 329-44[Medline].
-
Kaptan K, Beyan C, Ural AU: Helicobacter pylori--is it a novel causative agent in Vitamin B12 deficiency? Arch Intern Med 2000 May 8; 160(9): 1349-53[Medline].
-
Karlsson FA, Burman P, Loof L: Enzyme-linked immunosorbent assay of H+,K+-ATPase, the parietal cell antigen. Clin Exp Immunol 1987 Dec; 70(3): 604-10[Medline].
-
Karnaze DS, Carmel R: Neurologic and evoked potential abnormalities in subtle cobalamin deficiency states, including deficiency without anemia and with normal absorption of free cobalamin. Arch Neurol 1990 Sep; 47(9): 1008-12[Medline].
-
Katsaros VK, Glocker FX, Hemmer B, Schumacher M: MRI of spinal cord and brain lesions in subacute combined degeneration. Neuroradiology 1998 Nov; 40(11): 716-9[Medline].
-
Kieburtz KD, Giang DW, Schiffer RB, Vakil N: Abnormal vitamin B12 metabolism in human immunodeficiency virus infection. Association with neurological dysfunction. Arch Neurol 1991 Mar; 48(3): 312-4[Medline].
-
Kinsella LJ, Green R: 'Anesthesia paresthetica': nitrous oxide-induced cobalamin deficiency. Neurology 1995 Aug; 45(8): 1608-10[Medline].
-
Kirke PN, Molloy AM, Daly LE: Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 1993 Nov; 86(11): 703-8[Medline].
-
Krumholz A, Weiss HD, Goldstein PJ, Harris KC: Evoked responses in vitamin B12 deficiency. Ann Neurol 1981 Apr; 9(4): 407-9[Medline].
-
Larner AJ, Zeman AZ, Allen CM: MRI appearances in subacute combined degeneration of the spinal cord due to vitamin B12 deficiency. J Neurol Neurosurg Psychiatry 1997 Jan; 62(1): 99-100[Medline].
-
Lassen HCA, Henricksen E, Neukirch F: Treatment of tetanus: severe bone marrow depression after prolonged nitrous oxide anesthesia. Lancet 1956; 1: 527-530.
-
Layzer RB: Myeloneuropathy after prolonged exposure to nitrous oxide. Lancet 1978 Dec 9; 2(8102): 1227-30[Medline].
-
Lehmann M, Gottfries CG, Regland B: Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord 1999 Jan-Feb; 10(1): 12-20[Medline].
-
Leichtenstein O: Uber progressive perniciose anamie bei tabeskranken. Deutsche Medizinische Wochenschrift 1884; 10: 849.
-
Lester-Smith E: Purification of antipernicious amaemia factors from liver. Nature 1948; 161: 638-639.
-
Lichtheim L: Zur kenntniss der perniziosen anamie. Muchen. Schweiz med wochenschr 1887; 34: 300.
-
Lindenbaum J, Rosenberg IH, Wilson PW, et al: Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994 Jul; 60(1): 2-11[Medline].
-
Lindenbaum J, Savage DG, Stabler SP: Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 1990 Jun; 34(2): 99-107[Medline].
-
Magnaghi V, Veber D, Morabito A: Decreased GFAP-mRNA expression in spinal cord of cobalamin-deficient rats. FASEB J 2002; 16: 1820-1822[Medline].
-
Marie RM, Le Biez E, Busson P, et al: Nitrous oxide anesthesia-associated myelopathy. Arch Neurol 2000 Mar; 57(3): 380-2[Medline].
-
McCombe PA, McLeod JG: The peripheral neuropathy of vitamin B12 deficiency. J Neurol Sci 1984 Oct; 66(1): 117-26[Medline].
-
Metz J: Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr 1992; 12: 59-79[Medline].
-
Metz J: Pathogenesis of cobalamin neuropathy: deficiency of nervous system S-adenosylmethionine? Nutr Rev 1993 Jan; 51(1): 12-5[Medline].
-
Minnich W: Kenntnis der im Verlaufe der perniciosen anamie beobachteten spinalerkrankungen. Zeitschrift fur Klinische Medizin 1892; 21: 264-314.
-
Minot GR, Murphy WP: Treatment of pernicious anemia by a special diet. JAMA 1926; 87: 470-476.
-
Ozer EA, Turker M, Bakiler AR: Involuntary movements in infantile cobalamin deficiency appearing after treatment. Pediatr Neurol 2001 Jul; 25(1): 81-3[Medline].
-
Paltiel O, Falutz J, Veilleux M: Clinical correlates of subnormal vitamin B12 levels in patients infected with the human immunodeficiency virus. Am J Hematol 1995 Aug; 49(4): 318-22[Medline].
-
Pant SS, Asbury AK, Richardson EP Jr: The myelopathy of pernicious anemia. A neuropathological reappraisal. Acta Neurol Scand 1968; 44: Suppl 5:1-36[Medline].
-
Penix LP: Ischemic strokes secondary to vitamin B12 deficiency-induced hyperhomocystinemia. Neurology 1998 Aug; 51(2): 622-4[Medline].
-
Perros P, Singh RK, Ludlam CA, Frier BM: Prevalence of pernicious anaemia in patients with Type 1 diabetes mellitus and autoimmune thyroid disease. Diabet Med 2000 Oct; 17(10): 749-51[Medline].
-
Platica O, Janeczko R, Quadros EV: The cDNA sequence and the deduced amino acid sequence of human transcobalamin II show homology with rat intrinsic factor and human transcobalamin I. J Biol Chem 1991 Apr 25; 266(12): 7860-3[Medline].
-
Postiglione A, Milan G, Ruocco A: Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer's dementia. A case-control study. Gerontology 2001 Nov-Dec; 47(6): 324-9[Medline].
-
Pruthi RK, Tefferi A: Pernicious anemia revisited. Mayo Clin Proc 1994 Feb; 69(2): 144-50[Medline].
-
Putnam JJ: A group of cases of systemic scleroses of the spinal cord, associated with diffuse collateral degeneration, occurring in enfeebled persons past middle life, especially in women: Studied with particular reference to etiology. J Nerv Ment Dis 1891; 16: 69.
-
Remacha AF, Cadafalch J: Cobalamin deficiency in patients infected with the human immunodeficiency virus. Semin Hematol 1999 Jan; 36(1): 75-87[Medline].
-
Renault F, Verstichel P, Ploussard JP: Neuropathy in two cobalamin-deficient breast-fed infants of vegetarian mothers. Muscle Nerve 1999 Feb; 22(2): 252-4[Medline].
-
Richmond J, Davidson S: Subacute combined degeneration of the spinal cord in non-Addisonian megaloblastic anaemia. Quarterly Journal of Medicine 1958; 27: 517-531.
-
Rickes EL, Brink NG, Koniuszy FR: Crystalline vitamin B 12. science 1948; 107: 396.
-
Robertson KR, Stern RA, Hall CD: Vitamin B12 deficiency and nervous system disease in HIV infection. Arch Neurol 1993 Aug; 50(8): 807-11[Medline].
-
Russell JSR, Batten FE, Collier J: Subacute combined degeneration of the spinal cord. Brain 1900; 23: 39.
-
Sacco RL, Roberts JK, Jacobs BS: Homocysteine as a risk factor for ischemic stroke: an epidemiological story in evolution. Neuroepidemiology 1998; 17(4): 167-73[Medline].
-
Sahenk Z, Mendell JR, Couri D: Polyneuropathy from inhalation of N2O cartridges through a whipped-cream dispenser. Neurology 1978 May; 28(5): 485-7[Medline].
-
Savage DG, Lindenbaum J, Stabler SP: Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 1994 Mar; 96(3): 239-46[Medline].
-
Savage DG, Lindenbaum J: Neurological complications of acquired cobalamin deficiency: clinical aspects. Baillieres Clin Haematol 1995 Sep; 8(3): 657-78[Medline].
-
Scalabrino G, Mutti E, Veber D: Increased spinal cord NGF levels in rats with cobalamin (vitamin B12) deficiency. Neurosci Lett 2006 Mar 27; 396(2): 153-8[Medline].
-
Scalabrino G, Carpo M, Bamonti F: High tumor necrosis factor-alpha [corrected] levels in cerebrospinal fluid of cobalamin-deficient patients. Ann Neurol 2004; 56: 886-890[Medline].
-
Scalabrino G, Corsi MM, Veber D: Cobalamin (vitamin B(12)) positively regulates interleukin-6 levels in rat cerebrospinal fluid. J Neuroimmunol 2002; 127: 37-43[Medline].
-
Schilling RF: Is nitrous oxide a dangerous anesthetic for vitamin B12-deficient subjects? JAMA 1986 Mar 28; 255(12): 1605-6[Medline].
-
Scott E: The prevalence of pernicious anemia in Britain. J Coll Gen Pract Res News 1960; 3: 80-4.
-
Scott JM: Folate and vitamin B12. Proc Nutr Soc 1999 May; 58(2): 441-8[Medline].
-
Selhub J, D'Angelo A: Hyperhomocysteinemia and thrombosis: acquired conditions. Thromb Haemost 1997 Jul; 78(1): 527-31[Medline].
-
Stabler SP, Allen RH, Fried LP, et al: Racial differences in prevalence of cobalamin and folate deficiencies in disabled elderly women. Am J Clin Nutr 1999 Nov; 70(5): 911-9[Medline].
-
Stabler SP, Allen RH, Savage DG: Clinical spectrum and diagnosis of cobalamin deficiency. Blood 1990 Sep 1; 76(5): 871-81[Medline].
-
Stacy CB, Di Rocco A, Gould RJ: Methionine in the treatment of nitrous-oxide-induced neuropathy and myeloneuropathy. J Neurol 1992 Aug; 239(7): 401-3[Medline].
-
Steiner I, Kidron D, Soffer D: Sensory peripheral neuropathy of vitamin B12 deficiency: a primary demyelinating disease? J Neurol 1988 Jan; 235(3): 163-4[Medline].
-
Sumner AE, Chin MM, Abrahm JL, et al: Elevated methylmalonic acid and total homocysteine levels show high prevalence of vitamin B12 deficiency after gastric surgery. Ann Intern Med 1996 Mar 1; 124(5): 469-76[Medline].
-
Surtees R: Biochemical pathogenesis of subacute combined degeneration of the spinal cord and brain. J Inherit Metab Dis 1993; 16(4): 762-70[Medline].
-
Tan SV, Guiloff RJ: Hypothesis on the pathogenesis of vacuolar myelopathy, dementia, and peripheral neuropathy in AIDS. J Neurol Neurosurg Psychiatry 1998 Jul; 65(1): 23-8[Medline].
-
Tefferi A, Pruthi RK: The biochemical basis of cobalamin deficiency. Mayo Clin Proc 1994 Feb; 69(2): 181-6[Medline].
-
Toh BH, van Driel IR, Gleeson PA: Pernicious anemia. N Engl J Med 1997 Nov 13; 337(20): 1441-8[Medline].
-
Tracey JP, Schiffman FJ: Magnetic resonance imaging in cobalamin deficiency. Lancet 1992 May 9; 339(8802): 1172-3[Medline].
-
van der Mooren MJ, Wouters MG, Blom HJ, et al: Hormone replacement therapy may reduce high serum homocysteine in postmenopausal women. Eur J Clin Invest 1994 Nov; 24(11): 733-6[Medline].
-
Victor M: Polyneuropathy due to nutritional deficiency and alcoholism. In: Dyck PJ, Thomas PK, Lambert EH, eds. Peripheral Neuropathy. Philadelphia, Pa: WB Saunders; 1975: 1030.
-
Wadia RS, Bandishti S, Kharche M: B12 and folate deficiency: incidence and clinical features. Neurol India 2000 Dec; 48(4): 302-4[Medline].
-
Wang HX, Wahlin A, Basun H: Vitamin B(12) and folate in relation to the development of Alzheimer's disease. Neurology 2001 May 8; 56(9): 1188-94[Medline].
-
Weir DG, Scott JM: Brain function in the elderly: role of vitamin B12 and folate. Br Med Bull 1999; 55(3): 669-82[Medline].
-
Wilhelm H, Grodd W, Schiefer U: Uncommon chiasmal lesions: demyelinating disease, vasculitis, and cobalamin deficiency. Ger J Ophthalmol 1993; 2(4-5): 234-40[Medline].
-
Woltmann HW: The nervous symptoms in pernicious anemia: an analysis of one hundred and fifty cases. American Journal of Medical Science 1919; 173: 400-9.
-
Wright JD, Bialostosky K, Gunter EW, et al: Blood folate and vitamin B12: United States, 1988-94. Vital Health Stat 11 1998 Dec; (243): 1-78[Medline].
-
Yao Y, Yao SL, Yao SS: Prevalence of vitamin B12 deficiency among geriatric outpatients. J Fam Pract 1992 Nov; 35(5): 524-8[Medline].
-
Yoo JH, Chung CS, Kang SS: Relation of plasma homocyst(e)ine to cerebral infarction and cerebral atherosclerosis. Stroke 1998 Dec; 29(12): 2478-83[Medline].
NOTE:
| Medicine is a constantly changing science and not all therapies are clearly established. New research changes drug and treatment therapies daily. The authors, editors, and publisher of this journal have used their best efforts to provide information that is up-to-date and accurate and is generally accepted within medical standards at the time of publication. However, as medical science is constantly changing and human error is always possible, the authors, editors, and publisher or any other party involved with the publication of this article do not warrant the information in this article is accurate or complete, nor are they responsible for omissions or errors in the article or for the results of using this information. The reader should confirm the information in this article from other sources prior to use. In particular, all drug doses, indications, and contraindications should be confirmed in the package insert.
FULL DISCLAIMER |
| Vitamin B-12 Associated Neurological Diseases excerpt |