Dosing & Uses
Dosage Forms & Strengths
tablets
- 5mg
- 10mg
- 15mg
- 25mg
injection, powder for reconstitution
- 50mg
- 100mg
- 200mg
- 350mg
- 500mg
injectable solution
- 10mg/mL
Methotrexate Overdose
Administer as soon as possible and within 24 hr methotrexate level is >10-6 M or 48 hour level is >9 x 10-7 M, the dose of leucovorin should be increased to 100 mg/m2 IV every 3 hours until methotrexate level is <10-8 M
Dosing considerations
- Hydration (3 L/day) and urinary alkalinization with sodium bicarbonate solution should be employed concomitantly; bicarbonate dose should be adjusted to maintain urine pH at 7.0 or greater
High Dose Methotrexate Rescue
10 mg/m² IV q6hr for 10 doses; starts 24 hours after beginning of methotrexate infusion
May give PO after 1st IV dose
Adjust dose as follows:
Normal methotrexate elimination
- Serum methotrexate level approximately 10 micromolar, 24 hr after administration, 1 micromolar at 48 hr, <0.2 micromolar at 72 hr
- Administer 15 mg PO, IM, or IV q6hr for 60 hr (10 doses starting at 24 hr after start of methotrexate infusion
Delayed late methotrexate elimination
- Serum methotrexate level remaining above 0.2 micromolar at 72 hr, and more than 0.05 micromolar at 96 hr after administration
- Continue 15 mg PO, IM, or IV q6hr, until methotrexate level <0.05 micromolar
Delayed early methotrexate elimination and/or evidence of acute renal injury
- Serum methotrexate level of 50 micromolar or more at 24 hr, or 5 micromolar or more at 48 hr after administration, or a 100% or greater increase in serum creatinine level at 24 hr after methotrexate administration (eg, increase from 0.5 mg/dL to a level of 1 mg/dL or more)
- Administer 150 mg IV q3hr until methotrexate level is <1 micromolar; then 15 mg IV q3hr until methotrexate level is <0.05 micromolar
Dosing considerations
- In presence of gastrointestinal toxicity, nausea or vomiting, administer leucovorin parenterally; do not administer leucovorin intrathecally; serum creatinine and methotrexate levels should be determined at least once daily
- Administration, hydration, and urinary alkalization (pH of 7.0 or greater) should be continued until methotrexate level is below 5 x 10-8 M (0.05 micromolar)
Megaloblastic Anemia Due to Folate Deficiency
Up to 1 mg daily; there is no evidence that doses >1 mg/day have greater efficacy than those of 1 mg; additionally, loss of folate in urine becomes roughly logarithmic as the amount administered exceeds 1 mg
Coadministration with Trimetrexate (Discontinued)
20 mg/m² IV/PO q6hr (for PO, round dose up to next 25 mg increment)
Dose adjustment for both trimetrexate & leucovorin may be necessary if hematologic toxicity occurs
Advanced Colorectal Carcinoma (with 5FU)
Recommended 20 mg/m² IV followed by 425 mg/m² fluorouracil
Dose reduction treatment pause may be necessary based on hematologic toxicity
Methanol Poisoning
1 mg/kg (50-70 mg adults) IV q4-6hr
Trimethoprim Toxicity
10 mg/m² PO q6hr
Other Indications & Uses
Bone marrow suppression due to folic acid antagonism
Dosage Forms & Strengths
tablets
- 5mg
- 10mg
- 15mg
- 25mg
injection, powder for reconstitution
- 50mg
- 100mg
- 200mg
- 350mg
- 500mg
injectable solution
- 10mg/mL
Methotrexate Overdose
Administer as soon as possible and within 24 hr methotrexate level is >10-6 M or 48 hour level is >9 x 10-7 M, the dose of leucovorin should be increased to 100 mg/m2 IV every 3 hours until methotrexate level is <10-8 M
Dosing considerations
- Hydration (3 L/day) and urinary alkalinization with sodium bicarbonate solution should be employed concomitantly; bicarbonate dose should be adjusted to maintain urine pH at 7.0 or greater
High Dose Methotrexate Rescue
10 mg/m² IV q6hr for 10 doses; starts 24 hr after beginning of methotrexate infusion
May give PO after 1st IV dose
Adjust dose as follows:
Normal methotrexate elimination
- Serum methotrexate level approximately 10 micromolar, 24 hr after administration, 1 micromolar at 48 hr, <0.2 micromolar at 72 hr
- Administer 15 mg PO, IM, or IV q6hr for 60 hr (10 doses starting at 24 hr after start of methotrexate infusion
Delayed late methotrexate elimination
- Serum methotrexate level remaining above 0.2 micromolar at 72 hr, and more than 0.05 micromolar at 96 hr after administration
- Continue 15 mg PO, IM, or IV q6hr, until methotrexate level <0.05 micromolar
Delayed early methotrexate elimination and/or evidence of acute renal injury
- Serum methotrexate level of 50 micromolar or more at 24 hr, or 5 micromolar or more at 48 hr after administration, or a 100% or greater increase in serum creatinine level at 24 hr after methotrexate administration (eg, increase from 0.5 mg/dL to a level of 1 mg/dL or more)
- Administer 150 mg IV q3hr until methotrexate level is <1 micromolar; then 15 mg IV q3hr until methotrexate level is <0.05 micromolar
Dosing considerations
- In presence of gastrointestinal toxicity, nausea or vomiting, leucovorin should be administered parenterally; do not administer leucovorin intrathecally; serum creatinine and methotrexate levels should be determined at least once daily
- Administration, hydration, and urinary alkalization (pH of 7.0 or greater) should be continued until methotrexate level is below 5 x 10-8 M (0.05 micromolar)
Megaloblastic Anemia Due to Folate Deficiency
Up to 1 mg daily; there is no evidence that doses > 1 mg/day have greater efficacy than those of 1 mg; additionally, loss of folate in urine becomes roughly logarithmic as the amount administered exceeds 1 mg
Trimethoprim Toxicity
10 mg/m² PO q6hr
Interactions
Interaction Checker
No Results
Contraindicated
Serious - Use Alternative
Significant - Monitor Closely
Minor
Contraindicated (0)
Serious - Use Alternative (1)
- trimethoprim
leucovorin decreases effects of trimethoprim by pharmacodynamic antagonism. Avoid or Use Alternate Drug. Monitor for trimethoprim treatment failure or decreased efficacy when coadministered with leucovorin, especially when used with sulfamethoxazole for Pneumocystis jiroveci pneumonia in patients who are HIV positive .
Monitor Closely (3)
- capecitabine
leucovorin increases effects of capecitabine by pharmacodynamic synergism. Use Caution/Monitor.
- fluorouracil
leucovorin increases toxicity of fluorouracil by pharmacodynamic synergism. Use Caution/Monitor.
- glucarpidase
glucarpidase will decrease the level or effect of leucovorin by increasing metabolism. Modify Therapy/Monitor Closely. Leucorvorin, reduced folates, and folate antimetabolites are substrates for glucarpidase (hydrolyzes glutamate residue from folic acid and antifolates)
Minor (0)
Adverse Effects
Frequency Not Defined
Diarrhea
Nausea
Vomiting
Stomatitis
Thrombocytosis
Anaphylactoid reaction
Wheezing
Urticaria
Anaphylactoid reactions
Warnings
Contraindications
Vitamin B12 deficiency anemia and pernicious anemia
Cautions
Hypersensitivity reactions including anaphylactoid reactions & urticaria
Risk of severe neurological complications in patients with undiagnosed anemia
Geriatric or debilitated patients receiving cotreatment with fluorouracil
May increase treatment failure of sulfamethoxazole-trimethoprim therapies
Formulations containing benzyl alcohol not to be used in infants
Thrombocytosis reported during intra-arterial infusion of methotrexate
In treatment of accidental overdosages of folic acid antagonists, IV leucovorin should be administered as promptly as possible; as the time interval between antifolate administration (eg, methotrexate) and leucovorin rescue increases, leucovorin’s effectiveness in counteracting toxicity decreases
In the treatment of accidental overdosages of intrathecally administered folic acid antagonists, do not administer leucovorin intrathecally (may be fatal)
Monitoring of serum methotrexate concentration is essential in determining optimal dose and duration of treatment with leucovorin; delayed methotrexate excretion may be caused by a third space fluid accumulation (ie, ascites, pleural effusion), renal insufficiency, or inadequate hydration; under such circumstances, higher doses of leucovorin or prolonged administration may be indicated
Doses higher than those recommended for oral use must be given IV; because of the benzyl alcohol contained in certain diluents used for reconstituting drug for Injection, when doses > 10 mg/m2 administered, reconstitute leucovorin calcium for Injection with sterile water for Injection, USP, and used immediately
Because of calcium content of leucovorin solution, no more than 160 mg of leucovorin should be injected intravenously per minute (16 mL of a 10 mg/mL, or 8 mL of a 20 mg/mL solution per minute)
Leucovorin enhances toxicity of 5-fluorouracil; when these drugs are administered concurrently in the palliative therapy of advanced colorectal cancer, the dosage of 5-fluorouracil must be lower than usually administered
Although toxicities observed in patients treated with combination of leucovorin plus 5-fluorouracil are qualitatively similar to those observed in patients treated with 5-fl uorouracil alone, gastrointestinal toxicities (particularly stomatitis and diarrhea) are observed more commonly and may be more severe and of prolonged duration in patients treated with the combination
Therapy with leucovorin and 5-fluorouracil must not be initiated or continued in patients who have symptoms of gastrointestinal toxicity of any severity until those symptoms have completely resolved
Patients with diarrhea must be monitored with particular care until diarrhea has resolved, as rapid clinical deterioration leading to death can occur; in an additional study utilizing higher weekly doses of 5-fluorouracil and leucovorin, elderly and/or debilitated patients were found to be at greater risk for severe gastrointestinal toxicity
Seizures and/or syncope reported rarely in cancer patients receiving leucovorin, usually in association with fluoropyrimidine administration, and most commonly in those with CNS metastases or other predisposing factors, however, a causal relationship has not been established
Parenteral administration is preferable to oral dosing if there is possibility that patient may vomit and not absorb the leucovorin; leucovorin has no effect on non-hematologic toxicities of methotrexate such as nephrotoxicity resulting from drug and/ or metabolite precipitation in the kidney
Pregnancy & Lactation
Pregnancy Category: C
Lactation: not known whether distributed in breast milk; use caution
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk. C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done. D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk. X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist. NA: Information not available.Pharmacology
Mechanism of Action
Serves as a cofactor supplement to counteract folic acid antagonists such as methotrexate; leucovorin is an active metabolite of folic acid
Displaces methotrexate from intracellular binding sites and restores the folate required for DNA/RNA synthesis
In methanol toxicity it serves as a tetrahydrofolate source to help the body eliminate the formic acid resulting from methanol's toxicity
Pharmacokinetics
Distribution: all body tissues, predominantly in liver
Metabolism: Rapidly converted to THF derivatives
Excretion: Urine (primarily); feces
Half-life elimination: 4-8hr
Peak plasma time: 2 hr (PO); 10 min (as folate); 1 hr (as tetrahydrofolate)
Administration
IV Incompatibilities
Additive: fluorouracil, trimetrexate
Syringe: droperidol, trimetrexate, fluorouracil
Y-site: amphotericin B cholesteryl sulfate, droperidol, foscarnet, NaHCO3
IV Compatibilities
Solution: compatible w/ most common fluids
Additive: cisplatin, cisplatin w/ floxuridine, floxuridine
Syringe: bleomycin, cisplatin, cyclophosphamide, doxorubicin, droperidol, fluorouracil, furosemide, heparin, methotrexate, metoclopramide, mitomycin, vinblastine, vincristine
Y-site (partial list): bleomycin, cisplatin, cyclophosphamide, fluorouracil(?), furosemide, heparin, linezolid, methotrexate, metoclopramide, mitomycin
IV Preparation
Reconstitute w/ BWI or SWI of 10 mg/mL or 20 mg/mL (for 350 mg vial)
Available in 10 mg/mL preservative-free solutions
Do not use benzyl alcohol-containing diluents for doses >10 mg/sq.meter
Reconstituted solution is stable for 7 d
Use immediately if reconstituted w/ preservative-free solution
IV/IM Administration
Infusion: not to exceed 160 mg/min
Images
| BRAND | FORM. | UNIT PRICE | PILL IMAGE |
|---|---|---|---|
| leucovorin calcium oral - | 15 mg tablet |  | |
| leucovorin calcium oral - | 10 mg tablet | 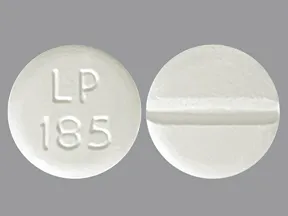 | |
| leucovorin calcium oral - | 5 mg tablet | 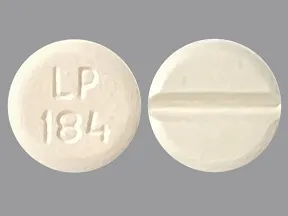 | |
| leucovorin calcium oral - | 10 mg tablet |  | |
| leucovorin calcium oral - | 25 mg tablet | 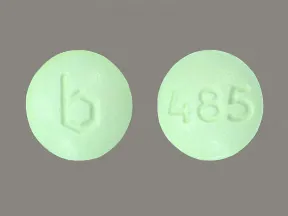 | |
| leucovorin calcium oral - | 25 mg tablet | 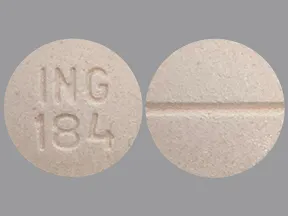 | |
| leucovorin calcium oral - | 5 mg tablet |  | |
| leucovorin calcium oral - | 5 mg tablet |  | |
| leucovorin calcium oral - | 25 mg tablet |  | |
| leucovorin calcium oral - | 10 mg tablet |  | |
| leucovorin calcium oral - | 15 mg tablet |  | |
| leucovorin calcium oral - | 5 mg tablet |  | |
| leucovorin calcium oral - | 15 mg tablet |  | |
| leucovorin calcium oral - | 5 mg tablet |  | |
| leucovorin calcium injection - | 350 mg vial |  | |
| leucovorin calcium injection - | 100 mg vial |  | |
| leucovorin calcium injection - | 200 mg vial |  | |
| leucovorin calcium injection - | 200 mg vial |  | |
| leucovorin calcium injection - | 350 mg vial |  | |
| leucovorin calcium injection - | 100 mg vial |  | |
| leucovorin calcium injection - | 50 mg vial |  | |
| leucovorin calcium injection - | 50 mg vial |  | |
| leucovorin calcium injection - | 200 mg vial |  | |
| leucovorin calcium injection - | 500 mg vial |  | |
| leucovorin calcium injection - | 100 mg vial |  | |
| leucovorin calcium injection - | 200 mg vial |  | |
| leucovorin calcium injection - | 350 mg vial |  | |
| leucovorin calcium injection - | 500 mg vial |  | |
| leucovorin calcium injection - | 350 mg vial |  | |
| leucovorin calcium injection - | 200 mg vial |  | |
| leucovorin calcium injection - | 100 mg vial |  | |
| leucovorin calcium injection - | 50 mg vial |  |
Copyright © 2010 First DataBank, Inc.
Patient Handout
leucovorin calcium oral
LEUCOVORIN - ORAL
(lew-ko-VORE-in)
USES: This medication is used to treat or prevent serious blood cell disorders (such as thrombocytopenia, neutropenia, anemia) caused by certain drugs (folic acid antagonists such as methotrexate, trimethoprim, pyrimethamine).
HOW TO USE: Take this medication by mouth with or without food as directed by your doctor, usually every 6 hours or once daily.The dosage is based on your medical condition and response to treatment.Use this medication regularly to get the most benefit from it. To help you remember, take it at the same time(s) each day.Tell your doctor if you are unable to take this medication because of nausea/vomiting. You may need to be switched to the injection form of this medication.
SIDE EFFECTS: Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS: Before taking leucovorin, tell your doctor or pharmacist if you are allergic to it; or to levoleucovorin; or to folic acid or folinic acid; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: certain anemias (due to vitamin B12 deficiency).Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).During pregnancy, this medication should be used only when clearly needed. Discuss the risks and benefits with your doctor.It is unknown if this medication passes into breast milk. Consult your doctor before breastfeeding.
DRUG INTERACTIONS: Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Leucovorin is very similar to levoleucovorin. Do not use medications containing levoleucovorin while using leucovorin.
OVERDOSE: If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call 1-800-222-1222. Canada residents can call 1-844-764-7669.
NOTES: Do not share this medication with others.Lab and/or medical tests (such as kidney function, complete blood count, folic acid antagonist blood levels) should be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.
MISSED DOSE: If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
STORAGE: Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Information last revised May 2024. Copyright(c) 2024 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Formulary
Adding plans allows you to compare formulary status to other drugs in the same class.
To view formulary information first create a list of plans. Your list will be saved and can be edited at any time.
Adding plans allows you to:
- View the formulary and any restrictions for each plan.
- Manage and view all your plans together – even plans in different states.
- Compare formulary status to other drugs in the same class.
- Access your plan list on any device – mobile or desktop.




